Carbon compounds
Hydrocarbons
These are the major class of organic compounds and they contain only carbon and hydrogen atoms.
· All other organic compounds are formed by replacing one or more hydrogen atoms of hydrocarbons by other atoms or groups. (hydrocarbon derivatives).
· All carbon compounds present in plants and animals are organic compounds. E.g. Carbohydrates, proteins, vitamins, nucleic acids, amino acids, fats and oils, natural polymers etc.
· Petroleum and coal are the major source of organic compounds (hydrocarbons).
Carbon compounds
Carbon is a unique element and it can form a large number of compounds due to the following reasons:
i) Tetravalency of carbon:
· In all of its compounds, the valency of carbon is four.
· Carbon has 4 electrons in its valency shell and requires 4 more electrons to complete the octet.
· So it attains the octet configuration by forming 4 covalent bonds.
ii) Ability to form single bond and multiple bonds:
Carbon can form single bond and multiple bond (double or triple bond) with itself and also with other elements like oxygen, nitrogen etc. This is possible by sp3, sp2 or sp hybridisation.
iii) Catenation:
Carbon shows catenation. Catenation is the self linking property of an element to form long chains and rings.
iv) Isomerism:
Carbon compounds can show isomerism.
It is the phenomenon in which compounds having same molecular formula but different structural formula or spatial arrangement of atoms.
Structural representation of organic compounds
An organic compound can be represented by the following ways:
1. Complete structural formula:
Here all the bonds between atoms are denoted by dashes (----). A single dash represents a single bond, a double dash represents a double bond and a triple dash represents a triple bond.
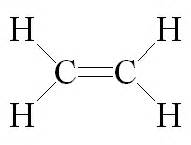 |
| Ethene |
 |
| Ethane |
2. Condensed structural formula:
Here the carbon-hydrogen bonds or all the bonds are omitted except the multiple bonds. It is a simplified representation of an organic compound.
E.g. ethane - CH₃CH₃, propane - CH₃CH₂CH₃,
butane - CH₃CH₂CH₂CH₃, ethene - CH₂=CH₂ etc.
3. Bond line representation:
Here carbon and hydrogen atoms are not shown and the lines representing carbon-carbon bonds are drawn in a zig-zag fashion.

The only atoms specifically written are oxygen, chlorine, nitrogen etc. The free terminals denote methyl (–CH3) groups.
4. Three-Dimensional Representation (Wedge Representation):
Here the structure of an organic molecule can be represented by using solid ( ) and dashed ( ) wedges.

Comments
Post a Comment